FDA provides Nod to SonarMed instrument For Monitoring Newborns

Houston-house biotech SonarMed has acquired FDA clearance to promote its non-invasive medical tool that may reveal, in actual-time, endotracheal tube place in newborn infants—and assist defend in opposition to suffocation.
the company in the past got regulatory acclaim for a version of the software used on adult patients when you consider that 2012. Executives didn’t divulge details on sales. “We wished to establish the necessity, and interest, and then to move on to the neonate and pediatric market, where want is extra urgent,” David Gunn, SonarMed’s vice president for technique and industry building, tells me
the problem is that endotracheal tubes, which can be inserted into the windpipe to help a affected person in respiratory, can move round and get clogged with mucus and other moisture. About 10 percent of the tubes come out fully, says Tom Bumgardner, SonarMed’s president and CEO. “it will lead to brain injury … 90 seconds for a child, two to a few minutes in adults,” he adds. “they are able to suffocate.”
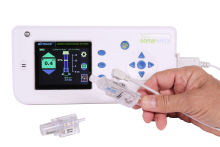
SonarMed’s device uses sonar expertise that sends sound pulses down the respiration tube. A sensor picks up echoes in the tube and relays that information to a display through which healthcare suppliers can see if the tube has moved or is blocked. The instrument can be utilized in each the intensive care and surgical environments, the corporate says.
within the ultimate yr, SonarMed has carried out pilots amongst adult sufferers on the university of Texas MD Anderson cancer heart in Houston, and it plans a second one at Stanford college. (SonarMed executives declined to supply the choice of sufferers in the studies or different details.)
“Having the device in patients allowed us to make some small tweaks within the product,” says Laura Lyons, the corporate’s vice chairman for medical, high quality, and regulatory affairs. “Our microphone know-how helps us overcome the difficulty” of clogging from mucus or humidity.
With the FDA clearance, SonarMed will begin marketing and ramping up production for its pediatric tool, in addition to the one designed for adults. SonarMed is focused on the highest 10 children’s hospitals in the united states firstly, Bumgardner says.
“For a neonate, a tiny baby, a small quantity of movement can also be catastrophic; it’s a model new lifestyles,” Lyons says. “We need to do the whole thing at all prices to give protection to it.”
SonarMed’s know-how came out of research completed via Jeffrey Mansfield, Eduardo Juan, and George Wodicka at Purdue college. the company, which used to be founded in 2005 in Carmel, IN, licensed the know-how from the Purdue analysis foundation and joined the analysis Valley Innovation storage, a co-working space situated at Texas A&M college in school Station, TX, in 2014 because it was once making use of to the the Texas rising technology Fund. (Upon entering office, Texas Gov. Greg Abbott abolished the fund—one of his marketing campaign promises—and SonarMed did not end up receiving money.)
the corporate has raised simply over $ 2.four million from a number of angel teams, including the crucial Texas Angel network, the Baylor Angel network, and the Texoma Angels, amongst others.
(11)