This New System Helps Bioengineering Happen 10,000 Times Faster
When researchers genetically engineer bacteria or yeast to solve a problem–creating bacteria that can eat plastic trash or make biofuel or insulin, or yeast that can make milk and cheese in a lab without a cow–one of the first steps can be the hardest: getting new DNA into a cell.
If a particular species hasn’t been engineered in the past, figuring out how to make its cells accept DNA can take months or even years. Actually inserting the DNA is equally tedious. Researchers typically use a manual process to pipette one sample of cells at a time into another chamber where the DNA is inserted, as they work to find a successful result among millions or billions of cells. A startup from MIT has new technology that can make that process 10,000 times faster.
“There’s been tremendous advances in writing DNA, tremendous advances in reading DNA, and tremendous advances in analyzing the data from the sequencing,” says Paulo Garcia, a research scientist at MIT and one of the cofounders of the startup, called Kytopen. “But the step where you actually put the DNA into the cells has been a bottleneck.”
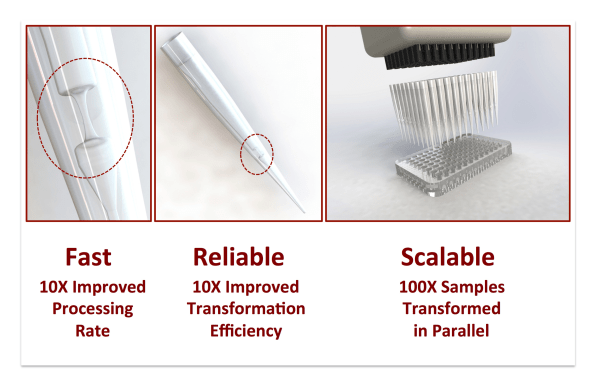
The technology uses a tiny fluid channel, roughly the width of a human hair, that narrows in the center and is built into the tip of a pipette. As cells flow through the channel, an electrical charge opens pores in the cells so DNA can enter; the shape of the channel minimizes the time that the electric charge is highest, so it’s less likely that a cell will be zapped so hard that it won’t survive the process.
With current techniques, working with one sample of cells at a time, someone might be able to process 50 samples in an hour. The new system can process 96 or more samples at once, each with millions of cells, generating billions of new variants in minutes. Within an hour, it can process as much as an older system could do in a year.
“In genetic engineering, it’s hard to sit down in advance at a computer and type out the DNA code that’s going to produce the function that you want . . . what I need to do is either try some designs, see how they perform, and iterate on that process until I finally get to what I want,” says Barry Canton, founder of Ginkgo Bioworks, a company that uses microbes to design perfumes, flavors, and other products. “Or, it would be preferable if I can right at the start make 10,000 different designs, try them all, and immediately find one that works.” Gingko, which has a huge facility filled with robotics, could become more efficient using the new technology; for a typical lab, it could make even more of a difference.
The new technology could make it possible to work with many more species of bacteria or yeast than have been used in the past, because it also solves the problem of how to create the unique conditions that make a specific species accept DNA. Of the millions of species of bacteria that are estimated to exist, for example, Kytopen’s founders say that the scientific community has been able to grow or harvest only about 1%, and of those, genetic engineering has only been possible on another tiny fraction.
If researchers can genetically engineer previously inaccessible species, they will likely discover new solutions. “Those organisms could hold inside of them the potential for creating new therapeutic proteins, or creating antibiotics, or perhaps creating enzymes that could be useful in food production or the derivation of biofuels,” says Cullen Buie, an associate professor of mechanical engineering at MIT and a cofounder of Kytopen. “There’s a tremendous amount of potential in biology that isn’t being realized because we can’t access it.”
In some ways, Buie says, it’s hard to predict what the impacts of the technology will be. “If you can do these processes so much faster, there are questions that people probably haven’t asked before because it would be too cumbersome. It’s really an enabling technology. I’d hate to sound like we’re trying to oversell it, but at the same time, if you could do a year’s worth of work in an hour, that would change the paradigm for how a lot of people conduct their research.”
The new startup has received seed funding from The Engine, an MIT-based accelerator that funds ideas that might find it challenging to get funding from typical venture capital firms. Kytopen is part of its first round of investment, along with startups such as Analytical Space, which develops systems that provide high-speed data from space, and iSee, which develops next-generation artificial intelligence for human-robotic collaboration. “I think pre-The Engine, a technology like ours would be likely to fall into this dangerous gap where it’s no longer fundamental research–the science is done–it’s not software, it’s not one of those other areas that’s getting venture funding these days,” Buie says. “It would be a shame for something like this to kind of die on the vine, but I think for a lot of technologies, that’s exactly what happens.”
Correction: This article formerly misspelled the name of the company. It is Kytopen, not Kytogen.
Fast Company , Read Full Story
(16)