COVID-19 saliva tests you can take at home have finally arrived
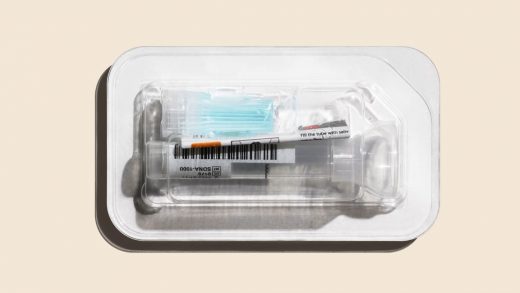
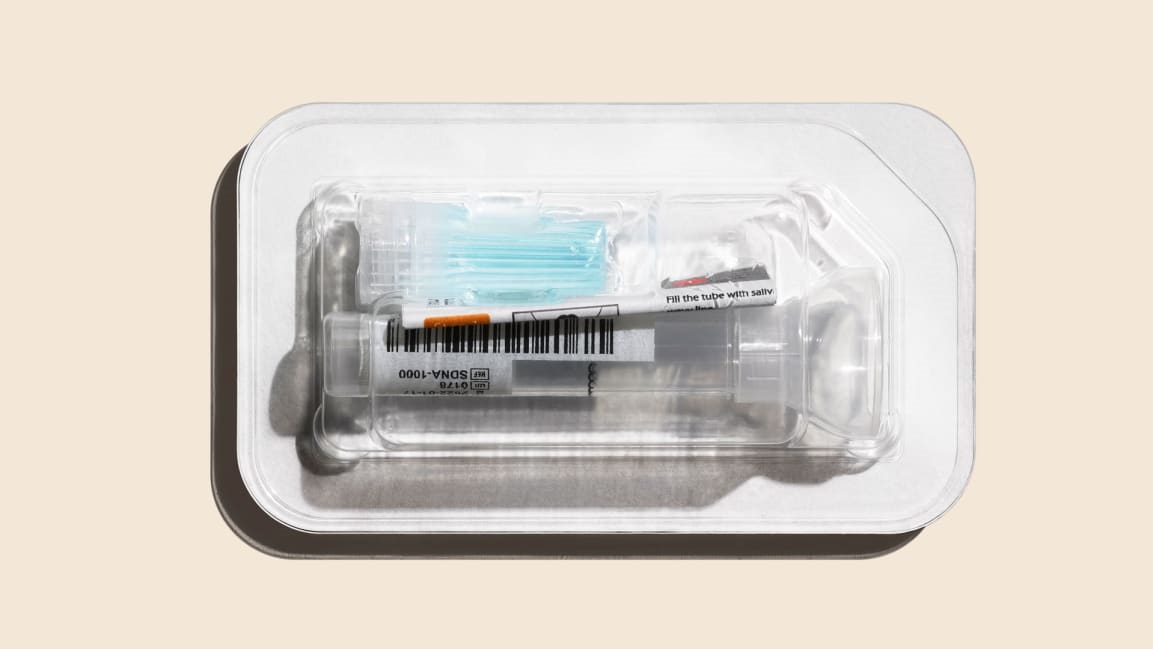
Telemedicine company Hims says it will start selling saliva tests for SARS-CoV-2 (the virus that causes the COVID-19 disease) that you can take at home from RUCDR Infinite Biologics, an organization within the Rutgers University Genomics Laboratory. Patients must be exhibiting symptoms to qualify for a test.
To get the test, people can go to Hims’s website and request a consultation. Applicants then fill out an intake form asking for symptoms and recent travel history. A doctor will review that information and determine whether or not a patient should receive a test. The process can be entirely asynchronous, meaning a patient does not have to interface with a physician in real time over video. However, some states require physicians to have live video visits. When the test comes by mail, patients spit into a tube and then send the sample off to the lab. Results are available two days later. Telehealth company Vault Health offers a similar process, whereby patients chart their symptoms in an online form before receiving a test, but the company supervises sample collection over video to ensure patients do it correctly.

RUCDR’s test is pricey. The Center for Medicare and Medicaid Services reimbursement rate for the test is $100, though Hims and Vault Health are selling the tests for $150 each. (A representative for Hims gave the following cost breakdown: $80 for RUCDR, $20 for the medical consultation and testing, and $50 for expedited shipping.)
Hims CEO Andrew Dudum says the company will start administering 2,000 tests per day. RUDCR says that it has the capacity to process 20,000 tests per day with a two-day turnaround. It plans to scale up to 50,000 tests per day. While the lab is currently working with Vault Health and Hims to administer the test, it may expand to other partners as well.
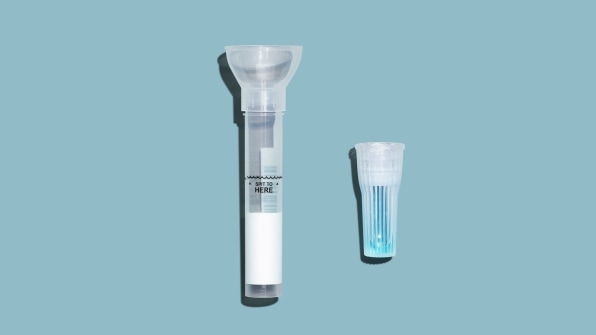
The Hims announcement comes days after the Food and Drug Administration awarded RUCDR an emergency-use authorization for its saliva test. The lab developed the test, which detects the coronavirus within a spit sample, in collaboration with Spectrum Solutions and Accurate Diagnostics. The FDA says the test may only be used on patients who are exhibiting symptoms. Unfortunately, the World Health Organization estimates that 80% of COVID-19 cases have mild or no symptoms.
Still, home testing holds big promise. Typically, nurses and doctors have to suit up in fresh protective gear every time they administer a COVID-19 test, which not only uses up protective equipment but also puts them at risk. Taking a saliva or nasal sample at home requires none of this. But since there have been myriad issues with COVID-19 testing, some are skeptical about home testing’s ability to deliver accurate results.
Testing for COVID-19 has been rife with problems since the virus landed in the United States. Initial tests issued from the Centers for Disease Control and Prevention were of such poor quality that they immediately set back testing efforts by weeks. Supply chain deficiencies created further setbacks as hospitals and laboratories ran short of materials needed to run tests, such as swabs and the chemicals used for analysis. In addition, the FDA has at times been unclear in its guidance, which has led to companies launching testing efforts, only to shutter them when the agency changed its rules. At-home testing kits, for example, first appeared in March but were quickly shut down when the FDA announced they were not approved. The FDA also allowed companies to develop and market antibody tests before obtaining an emergency-use authorization, which led companies to start selling unvalidated antibody tests. The FDA clamped down on these tests when some of them proved incredibly inaccurate. Now, companies must submit data proving the efficacy of their tests to the FDA.
These events have led to a very murky environment around testing. Even testing industry stalwarts have come under fire. Last month, Cleveland Clinic called out Abbott Labs’s rapid COVID-19 test for delivering too high a percentage of false negatives. Abbott Labs said any problems related to its test had to do with the way it was being used. For example, issues may arise if a doctor stores a patient sample while it is awaiting processing, since the machine is designed to process samples immediately after they’re taken.

These problems have resulted in concern about the reliability of home testing, especially given the potential for contamination or disturbance from the shipping process.
RUCDR’s saliva test is the second FDA-authorized at-home test to hit the market. In April, LabCorp received an emergency-use authorization for a nasal swab test called Pixel. The Pixel test is less invasive than the more typical nasopharyngeal test, where a doctor or nurse swabs the deep upper nasal cavity. However, the nasopharyngeal test is still regarded as the best option for testing when available. Neither has gone through the FDA’s typical approval process.
Dudum acknowledges these tests are not perfect. “If you get a negative result, it is possible that you still have the virus,” says Dudum. He says his physicians will be following guidance from the CDC as they work with patients to maintain social distancing and self-quarantine as needed even if they test negative. He says that Hims will be communicating positive case rates to public health officials, but that the company has limited ability to monitor false positives.
“We as a telemedicine platform in the middle aren’t really capable of doing so,” Dudum says. “It’s a constant effort from our lab partner Rutgers University and the FDA to be running sample set data to understand what those false positive rates are looking like.”
(34)