Johnson & Johnson gets FDA approval for heart treatments that don’t require X-rays
Johnson & Johnson gets FDA approval for heart treatments that don’t require X-rays

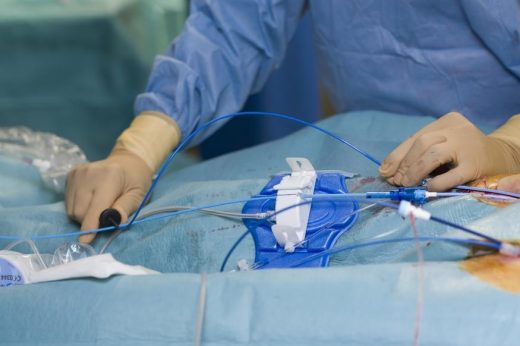
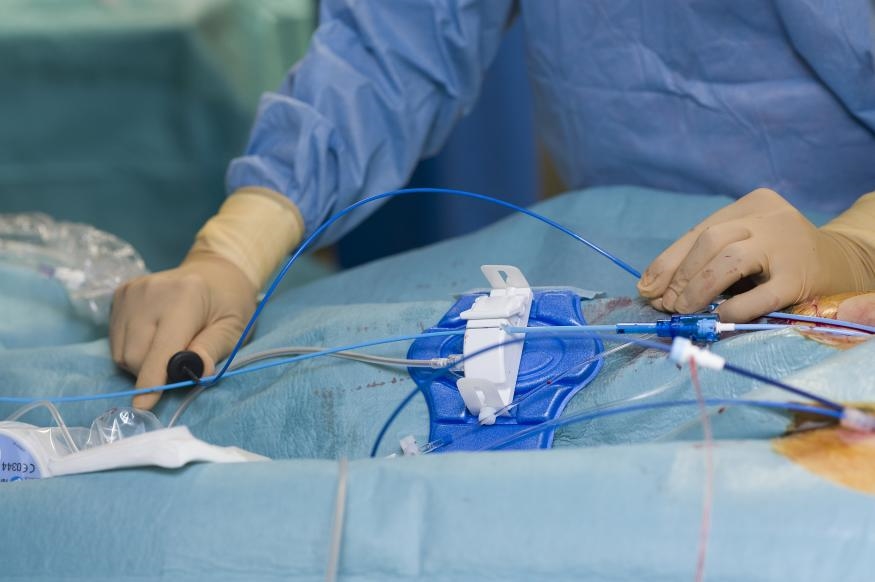
Johnson & Johnson’s Medical technology arm received FDA approval for a new workflow that will make it safer for medical professionals to treat atrial fibrillation, a condition that makes your heartbeat irregular and can cause stroke or heart failure. Several products developed by Biosense Webster, which is part of J&J MedTech, got the OK for a “zero fluoroscopy workflow” from the FDA, meaning live X-ray imaging will no longer be needed during catheter insertion procedures. Instead of using X-rays to insert Biosense catheters, medical professionals can now use ultrasound to guide treatments.
Using fewer X-rays, or fluoroscopy, lowers radiation exposure for both patients and medical professionals. Currently, doctors and medical staff who work in treatment rooms that specialize in treating relevant heart procedures often get too much exposure to radiation over time, which can lead to problems like eye issues, cancer, and bone injuries. This FDA approval helps address the recurring occupational hazard. Providers working in cath labs also won’t have to wear heavy protective gear like lead aprons anymore when applying the newly approved workflow, reducing the risk of long-term muscle and bone pain.
This move by the FDA marks the first and only approval of its kind. The thumbs up was based on data from clinical trials and research from the REAL AF Registry, or the real-world evidence registry in the electrophysiology field. The data backed how well the treatment works in real-life situations. The new method will only apply for Biosense products like the THERMOCOOL SMARTTOUCH SF catheter, the most commonly used ablation catheter, among others.
(8)