Vaccines for kids update: Biden announces distribution plan, pending CDC and FDA approval
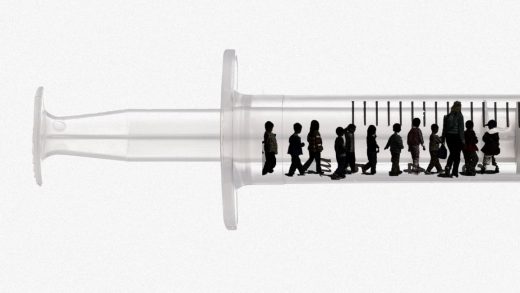
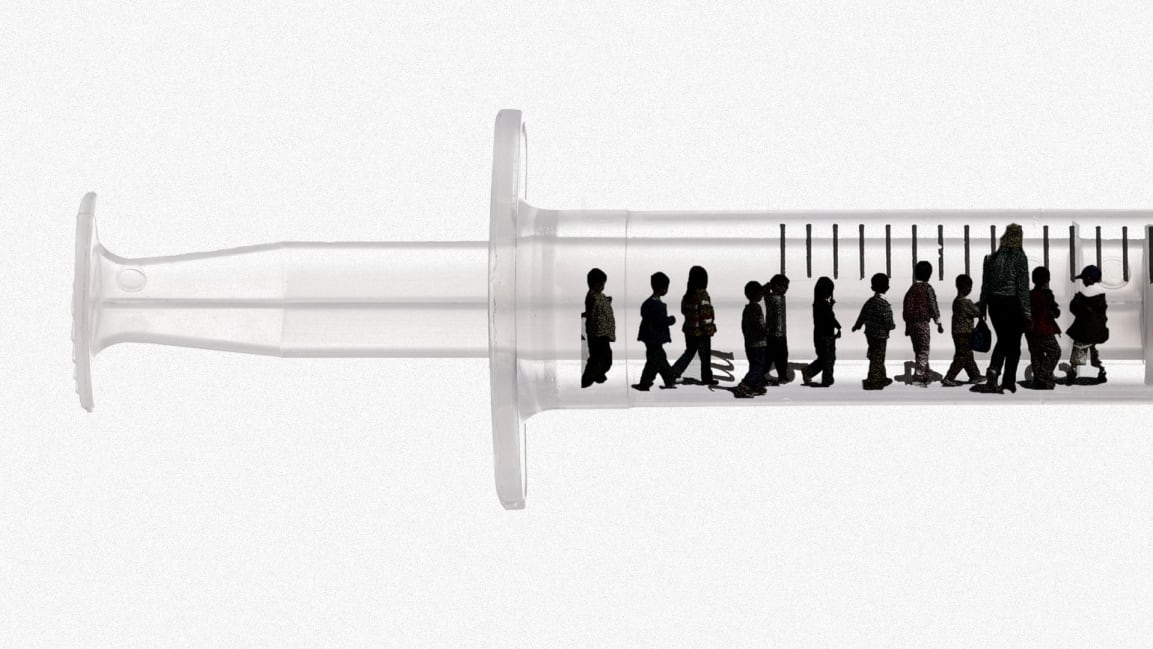
Federal health officials are poised to approve COVID-19 vaccines for children ages five to 11, so the Biden administration has unveiled how it plans to get the shots in those little arms.
This morning, the White House said that pending the OK from U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC), the vaccine will be administered at:
For schools looking to serve as vaccination sites, the federal government will help school districts find providers who can set up on-site vax clinics.
The Pfizer-BioNTech vaccine specially dosed and formulated for 5-11 year olds will come in 10-dose vials, in cartons of 10 vials each and with smaller needles. The vaccines can be refrigerated for 10 weeks and stored at ultracold temperature for six months.
The United States has an estimated 28 million 5- to 11-year-olds, according to the White House.
The U.S. Department of Health and Human Services also will launch a public education campaign for parents and guardians about vaccines for children of these ages.
“These steps will be critical in ensuring that we are staying ahead of the virus by keeping kids and families safe, especially those at highest risk,” the White House said in a written statement.
The FDA’s independent advisory committee meeting is scheduled for Tuesday and the CDC’s independent advisory committee meeting is November 2-3.
Currently, 189 million Americans are vaccinated, representing two out of three people eligible for the shots.
(23)